Analyse de l'innocuité, de la tolérance, de la pharmacocinétique, de l'occupation cible et de l'intervalle QT du nouvel inhibiteur de la BTK, Evobrutinib chez des volontaires en bonne santé
Clin Transl Sci . 2020 Mar;13(2):325-336. doi: 10.1111/cts.12713. Epub 2019 Nov 29.
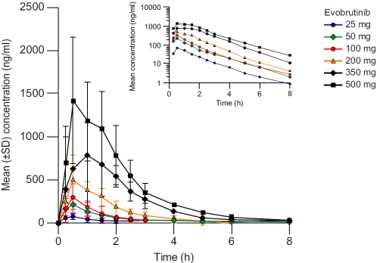
In this first in-human phase I, double-blind, placebo-controlled trial, the Bruton’s tyrosine kinase (BTK) inhibitor evobrutinib was well-tolerated, showed linear and time-independent PK, induced long-lasting BTK inhibition, and was not associated with prolongation of QT/QTc interval in healthy subjects. Evobrutinib is an oral BTK inhibitor that has demonstrated efficacy in pre-clinical and autoimmune disease models. BTK is a key regulator of B cell receptor and Fc receptor signalling, and a rational therapeutic target for autoimmune diseases. This study examined the safety, PK, and target occupancy of evobrutinib following single and multiple dose escalations, in subjects aged 18-55 years in good general health. TEAEs were mostly mild, occurring in 25% of subjects after single dosing, and 48.1% after multiple dosing. Absorption was rapid (time to reach maximum plasma concentration (Tmax) ~ 0.5 hour), half-life short (~ 2 hours), and PK dose-proportional, with no accumulation or timedependency on repeat dosing. High and sustained BTK occupancy was observed after single and multiple dosing, indicating slow turnover of BTK protein in vivo. There was no impact of evobrutinib concentration on corrected QT. The authors concluded evobrutinib was suitable for investigation in autoimmune disease.