Efficacy and safety of apremilast in paediatric patients with moderate-to-severe plaque psoriasis: 52-week results from SPROUT randomised control trial
Br J Dermatol 2025;193:1120–1127 Doi: 10.1093/bjd/ljaf305
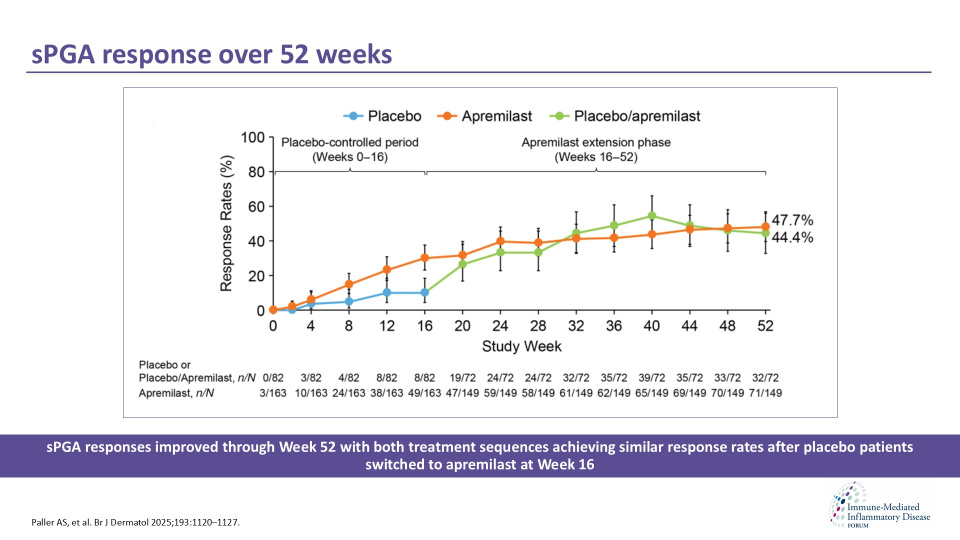
Paller et al. evaluated the long-term efficacy and safety of apremilast in paediatric patients with moderate-to-severe plaque psoriasis through the 52-week Phase III SPROUT trial. The study assessed whether clinical responses were sustained after the placebo-controlled period and whether outcomes differed between treatment sequences.
Data demonstrated that apremilast provided durable improvements in sPGA and PASI 75 responses through Week 52. Clinical benefits were comparable in patients who continued apremilast and those who switched from placebo at Week 16. Long-term treatment was well tolerated, with no new safety signals and low rates of serious or discontinuation-related TEAEs.