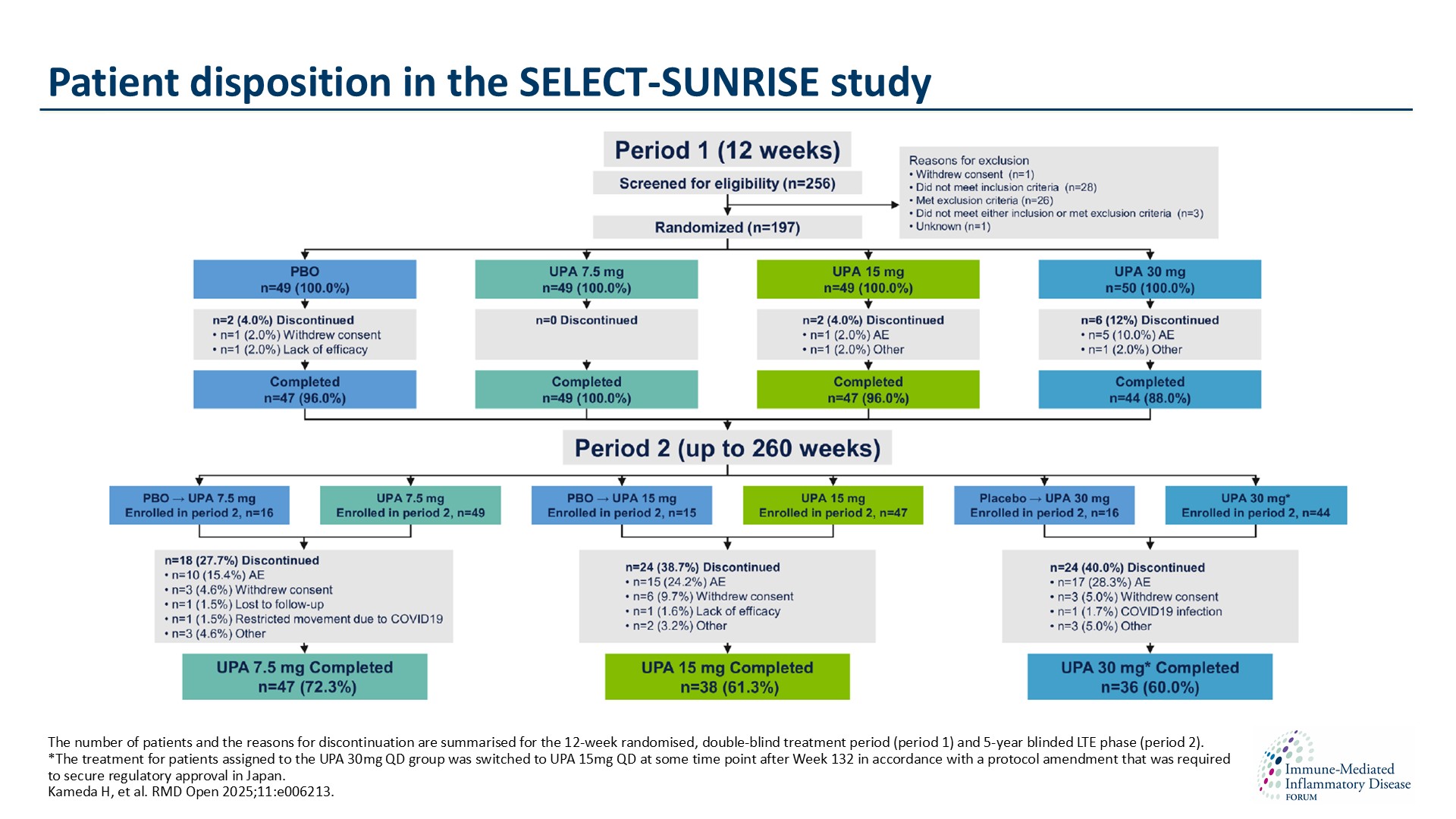
Long-term safety and efficacy of upadacitinib in Japanese patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying antirheumatic drugs: 5-year results from the SELECT-SUNRISE randomised controlled trial
RMD Open 2025;11:e006213 Doi: 10.1136/rmdopen-2025-006213
Kameda et al. reported that UPA treatment sustained efficacy with no new safety signals identified through 5 years of treatment and is a long-term treatment option for Japanese patients with RA and an inadequate response to csDMARDs. Authors present the full 5-year efficacy and safety data for upadacitinib obtained in the SELECT-SUNRISE study.
Several AEs, including serious infections, were numerically more frequent in the 15mg group than in the 7.5mg group, whereas some outcomes such as cardiac adverse events and malignancies were too infrequent to allow firm conclusions. The efficacy of UPA was generally maintained for 5 years in terms of low disease activity, clinical remission rates and patient-reported outcomes.