Efficacy and safety of guselkumab subcutaneous induction and maintenance in participants with moderately to severely active Crohn’s disease: Results from the Phase 3 GRAVITI study
Gastroenterology 2025;169:308–25 Doi: 10.1053/j.gastro.2025.02.033
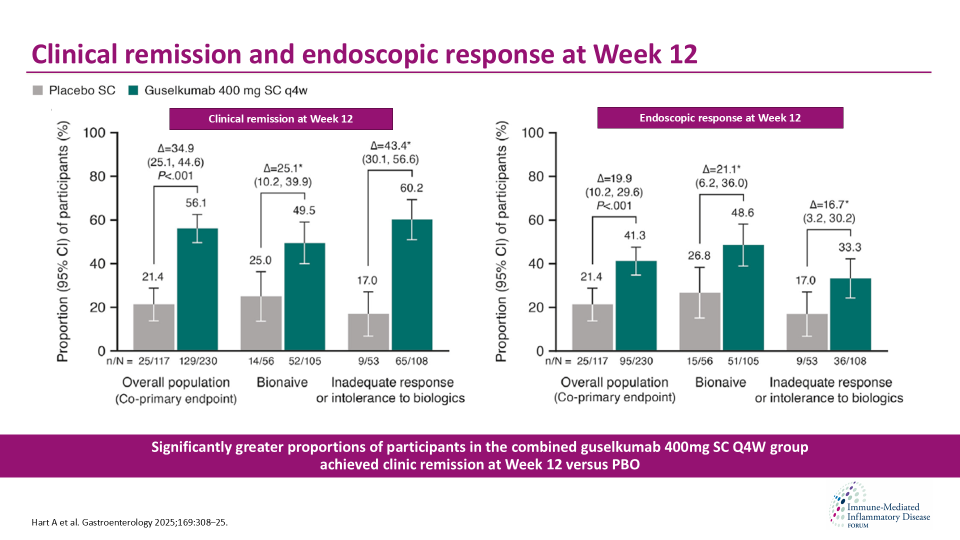
Results from the Phase 3 GRAVTI study by Hart et al. showed that SC induction followed by SC maintenance treatment with guselkumab resulted in superior clinical and endoscopic improvements in participants with moderately to severely active CD through 48 weeks compared with placebo. Hart et al. evaluated efficacy and safety of guselkumab SC induction followed by SC maintenance in participants with moderately to severely active CD in a treat-through design.
Safety results were consistent with the established safety profile of guselkumab in previously reported studies in CD, UC, and psoriatic diseases. Results by Hart et al. build on the GALAXI data and demonstrated that guselkumab induction efficacy can be achieved rapidly with both SC and IV dosing, providing options for administration that matches patient and health care provider preferences.