Ixekizumab and malignant neoplasms A pooled analysis of data from 25 randomized clinical trials
Merola et al. JAMA Dermatol 2025; 9:e252056
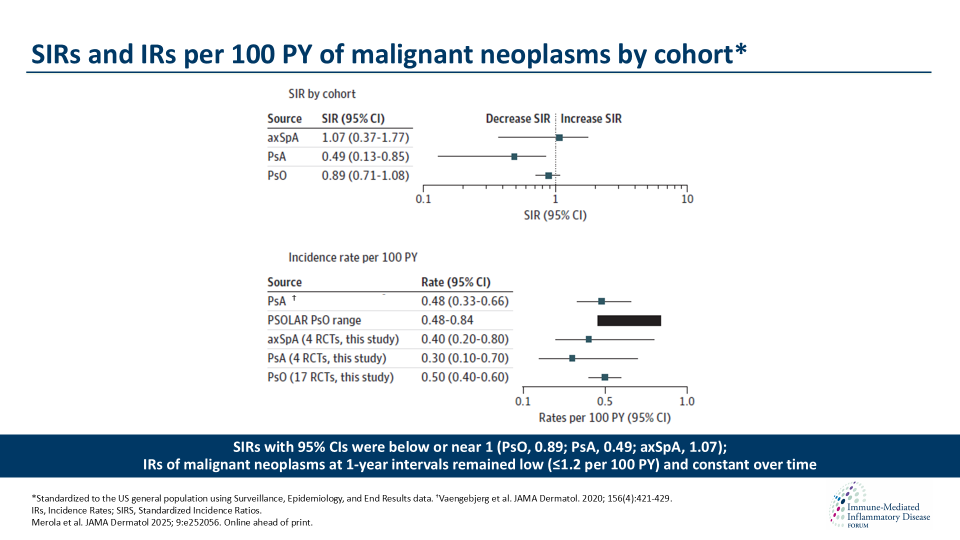
Merola et al. showed that the safety profile of ixekizumab (IXE) supports its long-term use in patients with PsO, PsA, or axSpA, without an increased risk for malignant neoplasm development. Merola et al. investigated the incidence rates of malignant neoplasms among patients with PsO, PsA, or axSpA who underwent long-term treatment with IXE, an IL- 17A antagonist.
This conclusion is substantiated by standardized incidence ratios of malignant neoplasms observed across the indications under study, which were comparable to those observed in the US general population. These findings should provide clinicians and patients with additional confidence in IXE as a reliable treatment option for long-term use in individuals with PsO, PsA, or axSpA.