Tocilizumab tedavisi sırasında oluşan majör kardiyovasküler advers olaylar için risk faktörlerinin değerlendirilmesi
Arthritis Rheumatol. 2015;67(2):372–380
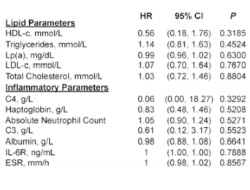
These retrospective post hoc analyses explore associations of baseline and on-treatment lipid levels, inflammation, and RA disease activity with risk for major adverse CV events (MACE) in tocilizumab (anti–IL-6 mAb)-treated RA patients. Data were pooled from 3986 adults with moderate to severe RA administered =1 dose of tocilizumab 4 or 8 mg/kg intravenously every 4 weeks in randomized controlled trials and extension studies. MACE was defined as definite or probable nonfatal MI, nonfatal stroke, or death from a CV cause. An association was observed between baseline total cholesterol/high-density lipoprotein ratio and increased risk for MACE, as in the general population. Risk for on-treatment MACE, however, was found to be associated with control of disease activity but not lipid changes. This analysis suggests that changes in measures of RA disease activity, but not necessarily lipid changes, are associated with incident MACE in tocilizumab-treated patients.