Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database
Ann Rheum Dis. 2021. Epub ahead of print. doi: 10.1136/annrheumdis-2021-221276.
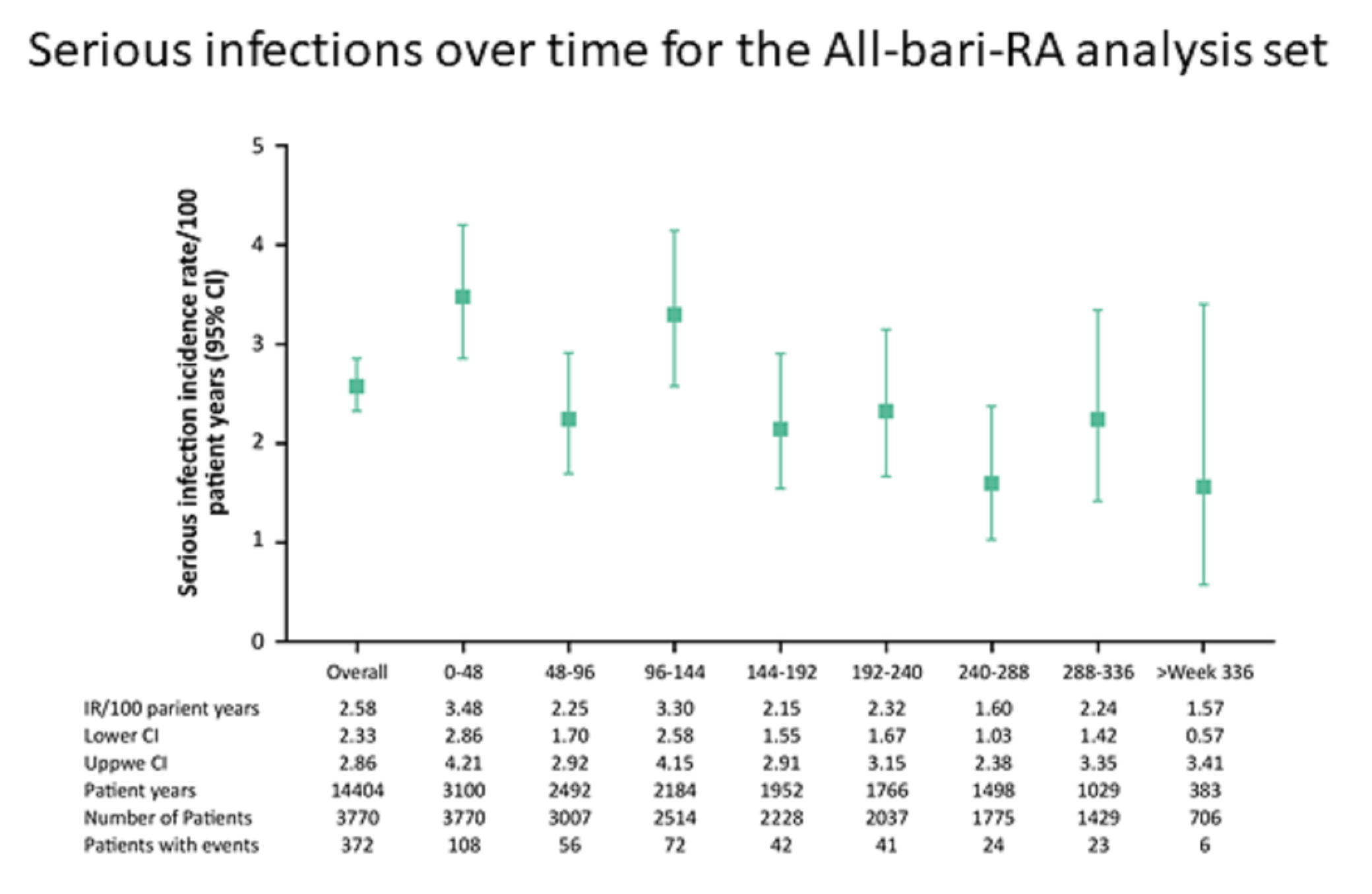
Analysis of data from the highest level of patient exposure to baricitinib across the spectrum of the RA population demonstrates that baricitinib maintained a similar safety profile to earlier analyses, with no new safety signals identified.Using integrated data from nine randomised controlled trials, Taylor, et al. assessed the safety of baricitinib 2 mg and 4 mg once-daily. Analysis of data from 3770 patients (median 4.6 years, up to 9.3 years) with active RA showed that baricitinib maintained a safety profile similar to that previously reported, with rates of safety events of special interest (including deaths, malignancies, MACE and DVT/PE) remaining stable through exposures up to 9.3 years.