Comparative effectiveness of first-line tumour necrosis factor inhibitor versus non-tumour necrosis factor inhibitor biologics and targeted synthetic agents in patients with rheumatoid arthritis: results from a large US registry study
Pappas DA,
St John G,
Etzel CJ,
Fiore S,
Blachley T,
Kimura T,
Punekar R,
Emeanuru K,
Choi J,
Boklage S,
Kremer JM
Ann Rheum Dis 2021;80:96–102.
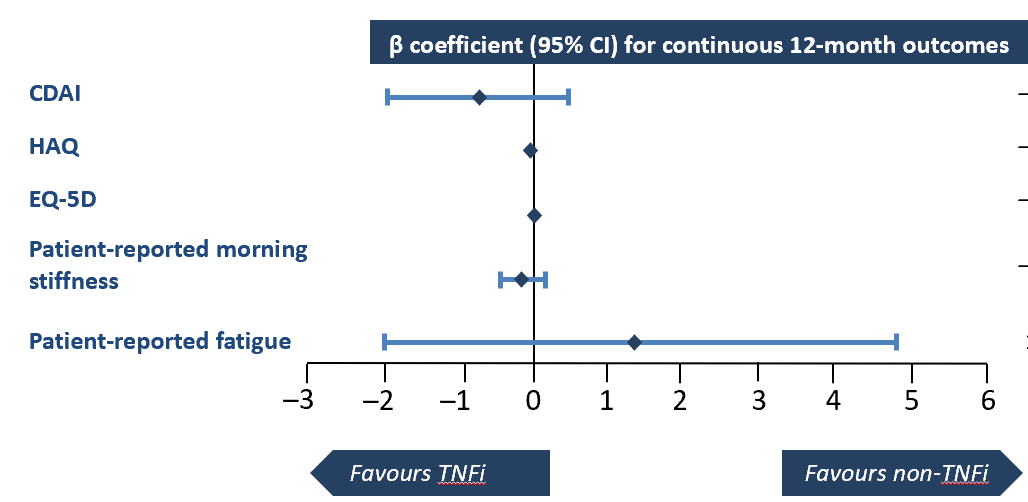
RA treatment guidelines recommend a treat-to-target approach guided by disease stage and treatment history, yet the optimal sequence of different treatment modalities has not been established. Data from Corrona – were used to evaluate the comparative effectiveness of TNFi versus non-TNFi bDMARDs and tsDMARDs as first-line treatment following csDMARD failure. Results support RA guidelines recommending individualised care based on clinical judgement and consideration of patient preference.The study included 4186 adult patients with a diagnosis of RA, a valid baseline CDAI greater than 2.8, and no prior b- or tsDMARDs. Outcomes including CDAI, 28-Joint Modified Disease Activity Score, PROs, and rates of anaemia were captured 1-year post-initiation. Groups were propensity score-matched to account for potential confounding. Post matching the groups appeared similar in terms of baseline characteristics. The results showed no significant differences between TNFi and non-TNFi treatment groups for binary outcomes, including achievement of LDA and remission. For anaemia, although the raw proportion with anaemia was not different pre- and post-matching, TNFi initiators had a lower crude incidence. This real-world, comparative study revealed limited differences in baseline characteristics and clinical outcomes between RA patients initiating treatment with TNFi versus non-TNFi following csDMARD failure.