Efficacité et Innocuité de l’Upadacitinib en Monothérapie Chez les Patients Méthotrexate-Naïfs Avec une Polyarthrite Rhumatoïde Modérément À Sévèrement Active (Select-early) : Un Essai Randomisé, en Double Aveugle, À Comparateur Actif, Multicentrique et Multinational
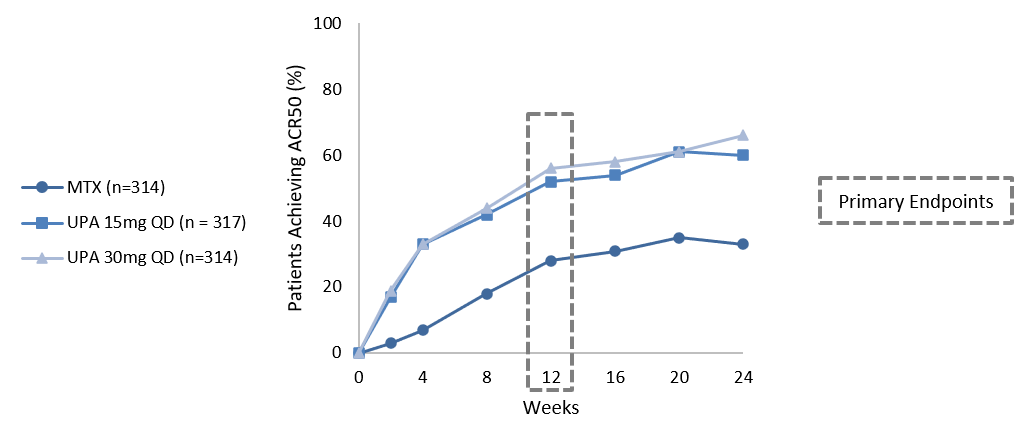
Upadacitinib monotherapy demonstrated superior clinical, radiographic, and patient-reported outcomes versus methotrexate in methotrexate-naïve RA patients.This 48-week double-blind active comparator study investigated upadacitinib monotherapy in patients with early RA, who were either methotrexate-naïve, or who had very limited exposure. 947 patients were randomised to once-daily upadacitinib 15 or 30 mg, or weekly methotrexate. Unusually, there were two separate primary endpoints, selected for the differing regulatory requirements of the FDA and EMA. For the FDA, the study recorded the proportion of patients achieving ACR50 at Week 12; and for the EMA, it was the proportion achieving DAS28-CRP at Week 24. Both primary endpoints were met by a significantly higher proportion of patients receiving either dose of upadacitinib versus methotrexate. ACR50 was met in 56%, 52% and 28%, and DAS28-CRP in 50%, 48% and 19% of 15 mg, 30 mg and methotrexate patients, respectively. These differences were apparent as early as the first visit at Week 2 and persisted through Week 24. Upadacitinib also demonstrated superiority across all secondary endpoints, and notably in various measures of LDA and remission, with significantly higher proportions at Week 24 compared with methotrexate.Treatment-emergent adverse events and laboratory variables were as expected, with no new safety signals. Through Week 24, the treatment-emergent adverse event rate was similar between methotrexate and upadacitinib 15 mg (65% and 64%), but slightly higher with the 30 mg dose (71%). Overall, the data are consistent with those previously reported in the upadacitinib Phase 3 trial programme. In summary, the authors concluded that once-daily upadacitinib monotherapy demonstrated superior clinical, radiographic, and patient-reported outcomes versus methotrexate in methotrexate-naïve RA patients at increased risk for structural damage.