Évaluation des réponses au vaccin contre le pneumocoque et le tétanos chez les patients atteints de polyarthrite rhumatoïde recevant du Baricitinib: Résultats d'une sous-étude sur l’essai de prolongation à long terme
Arthritis Res Ther. 2019 Apr 18;21(1):102
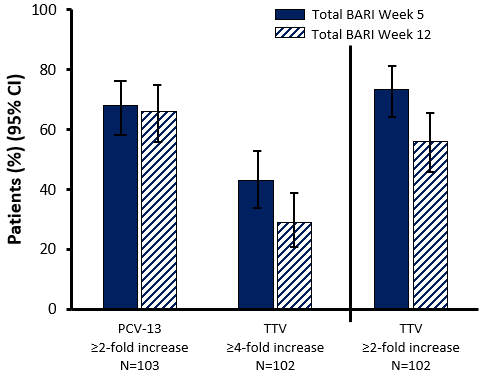
Approximately two thirds of long-term BARI treated patients achieved satisfactory humoral and functional responses to 13-serotype pneumococcal conjugate vaccine (PCV-13), whereas tetanus toxoid vaccine (TTV) responses were less robust. Both RA management guidelines and recommendations suggest vaccinating patients with RA against pneumococcal disease with PCV-13 and PPSV-23. The inhibition of the JAK mediated signal transduction pathways in RA treatment could diminish vaccine responses. Given the mechanism of action of BARI, this study evaluated the immunogenicity of the vaccines PCV-13 and TTV in RA patients receiving BARI treatment.Eligible RA patients receiving either BARI doses with or without concomitant MTX were enrolled into a phase 3 LTE trial (RA BEYOND) from USA and Puerto Rico. Patients were vaccinated with PCV-13 and TTV. The primary endpoints were the proportion of patients achieving a satisfactory humoral response for PCV-13 (≥ 2-fold increase in anti-pneumococcal antibody concentrations in ≥ 6 serotypes) and TTV (≥ 4-fold increase in anti-tetanus concentrations) at week 5. Secondary endpoints were geometric mean fold rise from baseline in pneumococcal serotype and tetanus antibodies at week 5. Primary endpoints were reached by 68% of patients for PCV-13 and 43% of patients for TTV at week 5. However, for TTV, 74% of patients achieved ≥2-fold concentration increase. Satisfactory patient responses were similar for PCV-13 regardless of BARI dose, concomitant corticosteroids, & SDAI response. Geometric mean concentrations for IgG were significantly higher compared to baseline for all serotypes at week 5 and 12. The anti-tetanus IgG antibody concentrations were also significantly higher at both week 5 and 12.Satisfactory pneumococcal humoral responses were demonstrated with both BARI doses, while not being affected by concomitant corticosteroid use, whereas TTV responses were less robust. Sustained functional responses (opsonophagocytic titers) of the pneumococcal antibodies were also demonstrated at week 5 and 12, including among some patients who lacked satisfactory response when measured by humoral titer. This suggests that protective responses can still be present even within individuals who lack “satisfactory” responses post-vaccination. Current guidelines recommend using both PCV-13 and PSV-23 to protect RA patients from S. pneumonia infection.