Profil d’innocuité du Baricitinib chez des patients Japonais atteints d’une polyarthrite rhumatoïde avec un temps de traitement d’une moyenne d’1,6 ans : analyse intégrée des essais de phases 2 et 3
Harigai M,
Takeuchi T,
Smolen JS,
Winthrop KL,
Nishikawa A,
Rooney TP,
Saifan CG,
Issa M,
Isaka Y,
Akashi N,
Ishii T,
Tanaka Y
Mod Rheumatol. 2019 Feb 20:1-23. DOI: 10.1080/14397595.2019.1583711
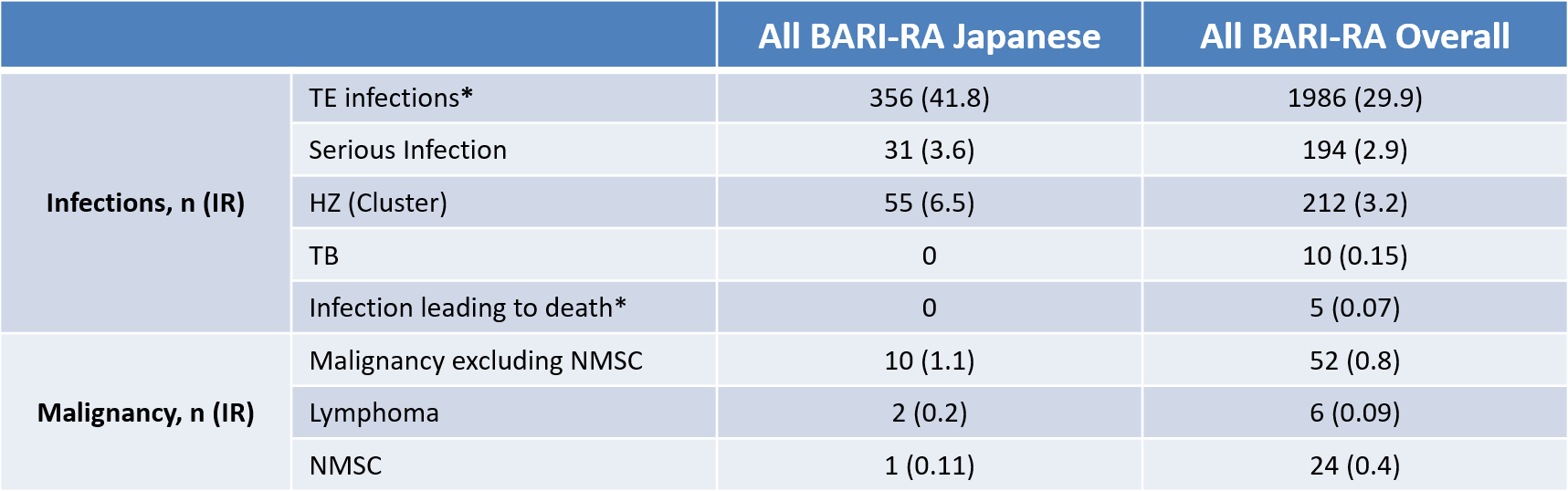
In this integrated analysis, BARI showed an acceptable safety profile in Japanese patients with up to 3.2 years of exposure. Other than incidences of herpes zoster (HZ), no major differences were noted with BARI safety in Japanese patients with RA, compared to the patients in the integrated database.BARI has previously demonstrated significant clinical efficacy and acceptable safety. Japanese patients who participated in the BARI clinical development programme, were comparable to those from the overall trial. This paper reports the integrated safety data across Japanese RA patients who participated in the BARI clinical development programme and compares this subset of patients with the overall integrated population.Data from 9 RA studies were initially integrated for assessment of safety. Japanese patients joined 6 of these trials, which were used to assess safety for the Japanese RA patients. IRs or exposure-adjusted IRs of AEs per 100 PY were calculated using data which included RA patients exposed to any BARI dose.The treatment emergent AE exposure-adjusted IR was higher in the Japanese patients (57.4/100 PY) compared to the overall patients (44.3/100 PY). Whereas, Japanese serious adverse event exposure-adjusted IR (9.3/100 PY) was similar to the overall patients (9.0/100 PY). No infections were found to lead to death, and no significant risk of malignancy in the Japanese RA patients compared to the overall patients were found. The most frequent serious adverse event reported were infections, with HZ (exposure-adjusted IR 1.5/100 PY) being the most commonly reported infection, but the events were considered manageable.