Efficacy and Safety of Filgotinib, a Selective Janus Kinase 1 Inhibitor, in Patients with Active Ankylosing Spondylitis (TORTUGA): Results from a Randomized, Placebo-controlled, Phase 2 Trial
Van der Heijde D,
Baraliakos X,
Gensler LS,
Maksymowych WP,
Tseluyko V,
Nadashkevich O,
Abi-Saab W,
Tasset C,
Meuleners L,
Besuyen R,
Hendrikx T,
Mozafarian N,
Liu K,
Greer JM,
Deodhar A,
Landewé R
Lancet 2018 Dec 1;392(10162):2378-2387. DOI 10.1016/S0140-6736(18)32463-2
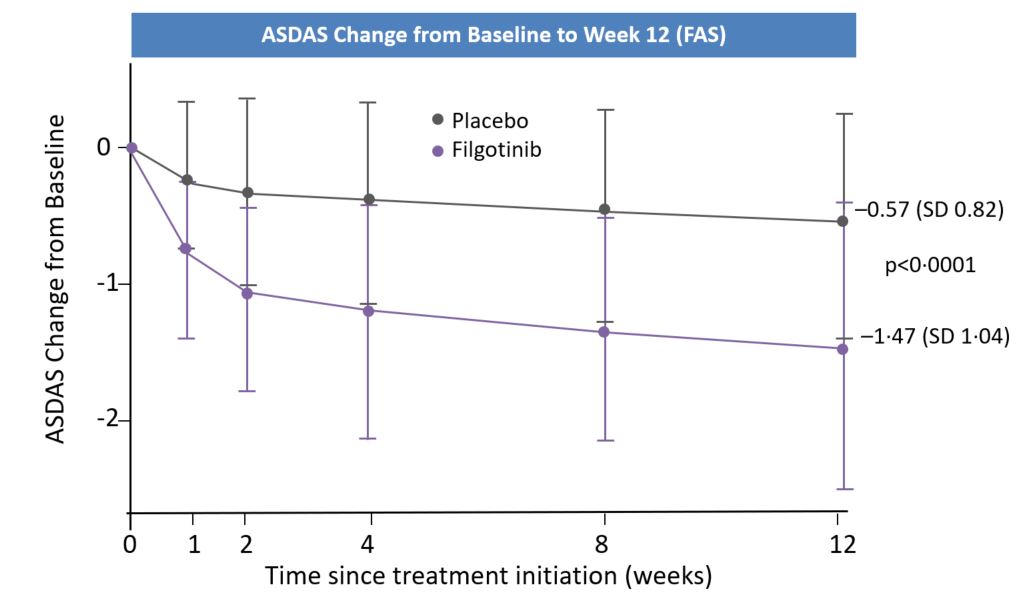
In this first clinical trial of filgotinib in patients with active AS, filgotinib significantly reduced disease activity, and the signs and symptoms of AS compared with placebo. The TORTUGA trial was a randomized, double-blind, placebo-controlled, Phase 2 trial, that enrolled 263 adult patients from 30 sites in seven countries. Patients with active AS and an inadequate response or intolerance to two or more NSAIDs were assigned 1:1 to receive filgotinib 200 mg or placebo once daily for 12 weeks. Following treatment, patients in the filgotinib group had a significantly greater reduction in disease activity, as assessed by change in ASDAS from baseline to week 12, than the placebo group. Significantly more patients experienced a clinically important or major improvement in ASDAS with filgotinib. Filgotinib also performed better than placebo in the secondary efficacy outcomes physical function, spinal mobility, peripheral arthritis, enthesitis, spinal and sacroiliac joint inflammation (assessed with MRI), fatigue, and quality-of-life measures. Filgotinib was well tolerated over 12 weeks of treatment with adverse events being mostly mild or moderate. The safety profile was consistent with findings from trials of filgotinib in patients with other conditions, including RA, Crohn’s disease, and PsA.The study supports the use of selective JAK-1 inhibition as a viable new treatment option for patients with active AS who have not responded to first-line pharmacological therapy with NSAIDs.