Effets Indésirables, Considérations Cliniques et Recommandations de Prise en Charge chez les Patients Atteints de Polyarthrite Rhumatoïde Traités par des Inhibiteurs de JAK
Exp Rev Clin Immunol 2018 Nov;14(11):945-956. DOI: 10.1080/1744666X.2018.1504678
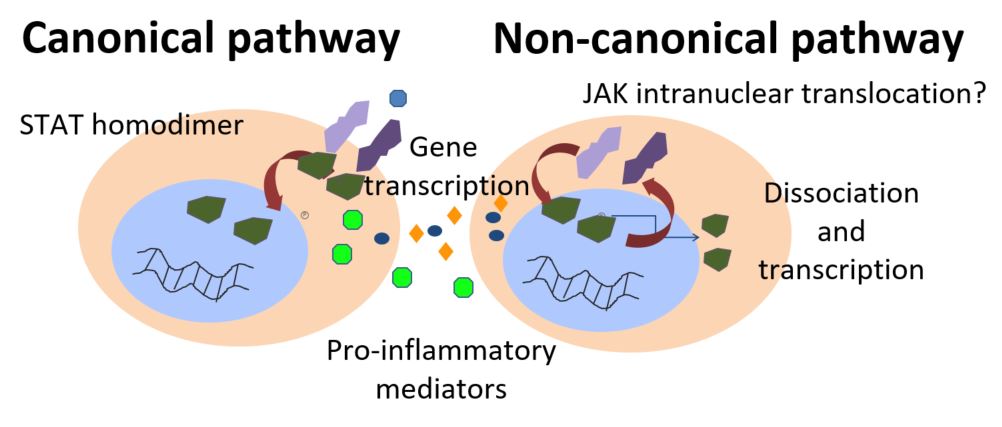
Janus kinase (JAK) inhibitors are efficacious in patients with moderate-to-severe RA and have a favourable safety profile. However adverse events (AE), in particular infections, are associated with the use of JAK inhibitors. This paper reviews the mechanism behind JAK inhibitors, the AEs associated with them, and provides consideration in the management of AEs in clinical practice. Data on two RA approved JAK inhibitors – tofacitinib (TOF) and baricitinib (BARI) – was obtained using PubMed, Medline and Cochrane Library database searches between 1999 and April 2018. Herpes zoster virus infection was a frequent adverse event in TOF and BARI clinical trials, with higher incidence noted than during conventional and anti-TNF treatment in RA patients. Hepatitis infections associated with JAK inhibitors are still debated, as published trials analysed excluded patients who screened positive for HBV or HCV. Short-term TOF treatment did not appear to increase Epstein-Barr virus load, but no data was available in BARI studies. Reactivation of latent tuberculosis has been reported in JAK inhibitor clinical trials, and patients should be screened for tuberculosis prior to starting these therapies. While patients with RA are at a greater risk of CV events than the general population1, studies of JAK inhibitors have not shown an increased risk of CV events. Gastrointestinal perforations and lymphoma cases have been reported rarely, but the authors suggested that there may be an intrinsic malignancy risk in RA patients, particularly those with high disease activity3.The authors concluded that because JAK inhibitors can be associated with a number of AEs, all clinicians, need to bear in mind these potential AEs to prevent and manage them. Pre-evaluation, including pre-screening is considered an important aspect of improving the standard of patient care and highlighting pre-dispositions in patients for certain AEs. 1.Boyer JF, et al. Arthritis Care Res 2012;64:872–880. 2.Dhillon S. Drugs 2017;77:1987–2001.3.Wilton KM, Matteson EL. Rheumatol Ther 2017;4:333–347.