Safety and Efficacy of Upadacitinib in Patients with Rheumatoid Arthritis and Inadequate Response to Conventional Synthetic Disease-Modifying Anti-Rheumatic Drugs (SELECT-NEXT): a Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial
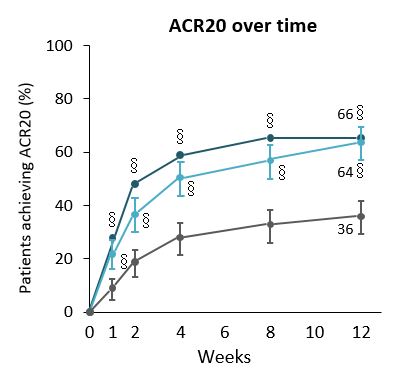
Patients with moderate-to-severe active RA had significant improvements in clinical signs and symptoms with upadacitinib (UPA) compared with placebo.In Phase 2 studies, UPA showed favourable efficacy when administered twice daily as an immediate-release formulation at doses of 6–12 mg in patients with active RA who had TNFi-IR.1,2 An extended-release formulation allowing once-daily (QD) administration was developed for Phase 3 studies. SELECT-NEXT was a double-blind, multicentre, Phase 3 study to assess the efficacy of UPA in patients with RA with csDMARD-IR. It involved a 12-week placebo-controlled, double-blind period followed by an ongoing double-blind extension up to five years. Here we report the first 12 weeks of data from SELECT-NEXT.661 patients were randomised to receive either: extended-release UPA 15 or 30 mg QD, or PBO for 12 weeks followed by extended-release UPA 15 or 30 mg QD. Primary endpoints were the proportions of patients achieving ACR20 and DAS28(CRP)<3.2 at Wk12. Key secondary endpoints included proportion of patients achieving ACR50, ACR70, DAS28(CRP)<2.6 and LDA (CDAI ≤10) at Wk12 and ACR20 at Wk1, and change from baseline in DAS28(CRP), HAQ-DI, SF-36 physical component score, FACIT-F scale, and duration of morning stiffness. Patients given either UPA dose achieved both primary endpoints at Wk12 and all prespecified key secondary endpoints. Patients with moderate-to-severe active RA had significant improvements in clinical signs and symptoms with UPA compared with PBO. UPA had a rapid onset of action, significantly more patients achieving ACR20 response with both doses of UPA at Wk1 versus PBO, and from Wk2 onwards for ACR50 and ACR70. Both doses of UPA were superior to PBO for stringent efficacy measures aligned with EULAR recommendations and treat-to-target strategy (LDA, DAS28(CRP)<2∙6 and CR), severity and duration of morning stiffness with both doses of UPA, and PROs. Most frequently reported AEs were nausea, nasopharyngitis, URTI and headache; more infections were reported for both doses of UPA than placebo.Overall, UPA had a favourable benefit-to-risk profile, although more patients taking UPA had infections or premature discontinuation due to AEs.1. Kremer JM, et al. Arthritis Rheumatol 2016;68:2867–77.2. Genovese MC, et al. Arthritis Rheumatol 2016;68:2857–66.