A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone
Arthritis & Rheumatism 2012; 64(4):970-81
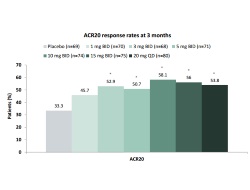
This study was one of two 24-week, phase 2b studies undertaken to characterise the efficacy and safety dose-response profile of the oral Janus kinase (JAK) inhibitor tofacitinib. Six doses of tofacitinib (20 mg daily and 1, 3, 5, 10 and 15 mg twice daily) and placebo were compared as add-on therapy in adults with active RA despite methotrexate (MTX) therapy. At week 12, ACR 20 response rates were significantly higher with all tofacitinib doses than with placebo (tofacitinib 45.7–58.1%; 33% placebo). ACR 20 response rates were significantly higher with tofacitinib than placebo at week 2, and responses were sustained for 24 weeks. Dose-related improvements in ACR 50/70 response rates, DAS28-CRP, HAQ-DI and SF-36 were statistically significant for all tofacitinib doses compared with placebo. Patients taking 15 mg tofacitinib twice daily reported the most adverse events; the most common adverse events up to week 12 included headache, diarrhoea, nausea, urinary and upper respiratory tract infections, nasopharyngitis, influenza, cough and liver enzyme abnormalities. The data in this study show that the addition of tofacitinib to background MTX is an effective treatment option for patients with active RA despite MTX; it also supported the decision to use 5 and 10 mg tofacitinib in phase 3 trials.
Slides coming soon