Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: Results of a phase III study
Arthritis Rheumatol. Vol. 67, No. 6, June 2015, pp 1424–1437
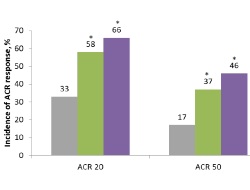
Sarilumab is a fully human anti-IL-6Rα mAb that binds membrane-bound and soluble human IL-6R with high affinity, blocking cis and trans IL-6-mediated signalling. This study (MOBILITY) is the first randomised, double-blind, placebo-controlled study to show that both 150 mg and 200 mg of sarilumab plus MTX, administered subcutaneously every 2 weeks, significantly improves signs and symptoms, physical function, and reduces radiographic progression in patients with moderate-to-severe RA. These data suggest that sarilumab holds promise as a treatment option for RA patients.