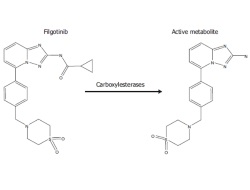
Pharmacokinetics and Pharmacokinetic/Pharmacodynamic Modeling of Filgotinib (GLPG0634), a Selective JAK1 Inhibitor, in Support of Phase IIB Dose Selection
Clin Pharmacokinet. 2015 Feb 14. [Epub ahead of print]
Two phase I, randomised, double-blind, placebo-controlled trials in healthy volunteers and one phase IIa proof-of-concept study in RA patients were used to evaluate single and multiple doses of filgotinib.
Results showed dose-dependent pharmacodynamic activity of combined filgotinib and its metabolite. Filgotinib was extensively and rapidly absorbed after single and repeated oral dosing in healthy volunteers. While PK/PD modelling and simulation showed a maximal pharmacodynamic effect achieved at daily dosing of 200 mg. This dose has now been selected as the highest dose in the phase IIb programme for filgotinib.