The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers
Mod J Clin Pharmacol. 2014 Dec;54(12):1354-61. doi: 10.1002/jcph.354.
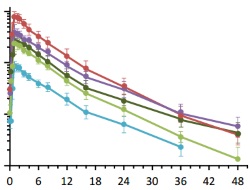
This report summarises the findings of the first-in-human and single ascending dose and multiple ascending doses studies of baricitinib. Two double-blind, randomised, and placebo-controlled studies were conducted to evaluate single ascending doses of 1–20 mg and multiple ascending doses of 2–20 mg QD and 5 mg BID for 10 or 28 days in healthy volunteers.
Baricitinib demonstrated a favourable pharmacokinetic and pharmacodynamics profile suitable for once-daily administration, with an overall safety profile supporting its further clinical development.