Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate
Ann Rheum Dis. 2015;74(2):333–340
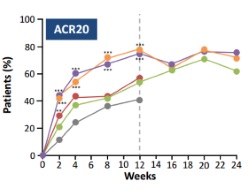
This phase IIb study was designed to investigate multiple doses and dosing regimens of baricitinib, for the treatment of patients with active RA with inadequate responses to MTX. At 12 weeks, 4 mg and 8 mg of baricitinib achieved similar efficacy in the primary efficacy endpoint ACR20, with significantly more patients in the combined baricitinib 4 and 8 mg groups meeting the criteria for ACR20 response at week 12 than placebo(76% vs 41%, p<0.001). Secondary efficacy endpoints showed similar significant benefits versus placebo, with all efficacy responses sustained or improved over the 24-week blinded period of the study. However, a mean decline in haemoglobin was observed in the 8 mg group consistent with a higher percentage of grade 1 abnormalities (≥10.0 g/dL to less than lower limit of normal) compared with all other groups.
The results of this study with baricitinib demonstrate that selective inhibition of JAK1 and JAK2 is effective and well tolerated in patients with active RA taking background MTX, with 4 mg baricitinib being considered the optimal dose for evaluation in phase III RA studies.