Tofacitinib Versus Methotrexate In Rheumatoid Arthritis
N Engl J Med. 2014;370(25):2377–2386.
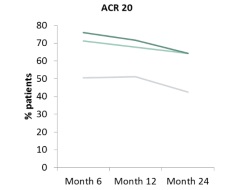
ORAL Start is the latest trial to be reported in the tofacitinib clinical development programme. It compares the use of tofacitinib monotherapy to MTX monotherapy, in RA patients who have had either no or a sub-therapeutic dose of MTX in the past. Nine hundred and fifty eight patients received either tofacitinib (5 mg or 10 mg) twice daily, or methotrexate at a dose that was incrementally increased to 20 mg per week over 8 weeks. The co primary efficacy endpoints were ACR 70 response, and mean change from baseline in the van der Heijde modified total Sharp score at 6 months. Secondary endpoints included these and other standard efficacy measures at various time points up to 2 years. After six months, clinical outcomes showed that twice as many patients in the tofacitinib groups achieved an ACR 70 response, compared to the MTX group. At the same time point, tofacitinib-treated patients showed significantly less progression from baseline in the modified total Sharp score compared to MTX. This significance was maintained out to 2 years. Similarly all other secondary efficacy endpoints showed significant differences in favour of tofacitinib at 6, 12 and 24 months. The safety profile was consistent with that reported in previous trials. Infection was the most common adverse event with herpes zoster occurring more frequently in the tofacitinib groups. Additionally, increases in creatinine levels, low-density and high-density lipoprotein cholesterol levels and reductions in neutrophil and lymphocyte counts were also recorded in the tofacitinib group.
Overall the study demonstrates that tofacitinib is more efficacious than MTX in this MTX naïve population with a safety profile that is consistent with previous studies of tofacitinib.