Filgotinib en Association avec le Méthotrexate ou en Monothérapie versus Méthotrexate en Monothérapie chez les Patients Atteints de Polyarthrite Rhumatoïde Active et Peu ou Pas Exposés au Méthotrexate : l'Essai de Phase 3, Randomisé et Contrôlé FINCH 3
Westhovens R,
Rigby WF,
van der Heijde D,
Ching DW,
Stohl W,
Kay J,
Chopra A,
Bartok B,
Matzkies F,
Yin Z,
Guo Y,
Tasset C,
Sundy JS,
Jahreis A,
Mozaffarian N,
Messina OD,
Landewé R BM,
Atsumi T,
Burmester GR
Ann Rheum Dis. 2021 Jan 15:annrheumdis-2020-219213.
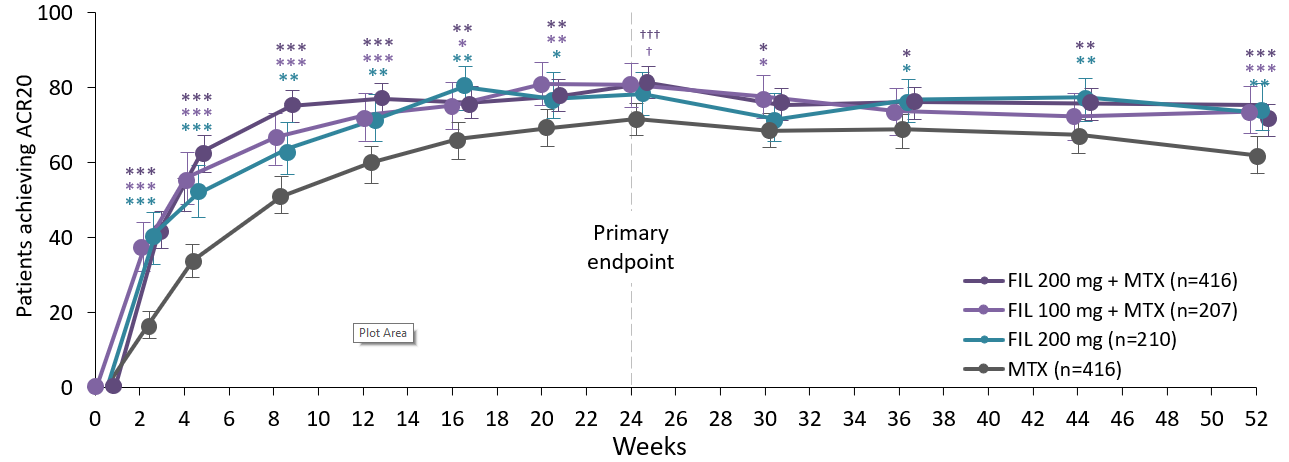
Filgotinib doses in combination with MTX have shown improved signs, symptoms and physical function in patients with RA and limited or no prior MTX exposure. FIL 200mg monotherapy did not have a superior ACR20 response rate versus MTX. This 52-week, phase 3 study evaluated FIL in 1252 patients with RA. Patients were randomised to FIL 200mg + MTX or FIL 100mg + MTX, FIL 200 mg monotherapy, or MTX monotherapy. The primary endpoint was the proportion patients achieving ACR20 at week 24. Safety was evaluated through AEs and laboratory assessments.ACR20 was achieved by 81% of patients receiving FIL 200mg + MTX vs 71% receiving MTX alone. Patients receiving FIL 200mg + MTX or FIL 100mg + MTX also had significant improvements in HAQ-DI, and DAS28 (CRP) <2.6 vs MTX at week 24. Least square mean changes from baseline in mTSS at week 24 was 0.13 with FIL 200mg + MTX and FIL 100mg + MTX, and −0.13 with FIL 200mg monotherapy vs 0.42 with MTX. Over 52 weeks, the safety of FIL was well tolerated, with acceptable safety compared with MTX.