Efficacité et Innocuité Comparées du Péficitinib 25, 50, 100 et 150 mg Chez les Patients Atteints de Polyarthrite Rhumatoïde Active : Une Méta-Analyse Bayésienne en Réseau d'Essais Contrôlés Randomisés
Clin Drug Investig .2020 Jan;40(1):65-72. doi: 10.1007/s40261-019-00863-9.
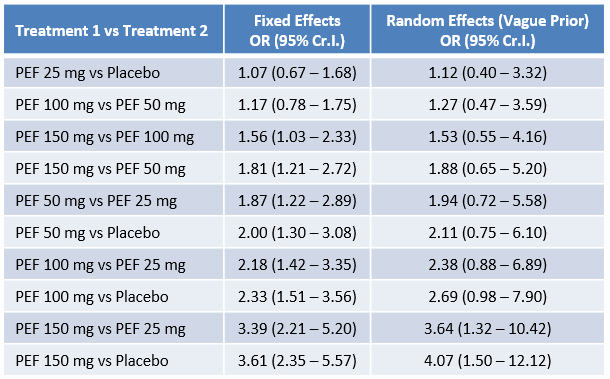
PEF 50, 100, and 150 mg once daily was effective in treating active RA, without causing a significant risk for AEs.Intracellular pathways, including JAK and Tyk-2, are critical for immune cell activation, pro-inflammatory cytokine production, and cytokine signaling. PEF has been developed for use in RA, but the comparative efficacy and safety of regimens and dosages has not been established. A Bayesian network meta-analysis was conducted to combine direct and indirect evidence to assess the relative efficacy and safety of peficitinib 25, 50, 100, 150 mg, or placebo in patients with active RA. Three trials involving 948 patients met the inclusion criteria. A dose-dependent response was observed for ACR20, with increasing efficacy at higher doses. When comparing the relative efficacy of peficitinib, ACR20 responses were significantly higher with 150 mg than placebo. This was confirmed with rank probability, which indicated that PEF 150 mg was likely to achieve the best ACR20 response rate. There were no dose-related increases in any safety outcomes, although PEF 50 and 25 mg were ranked as likely to be the safest treatments; however, the number of patients who experienced adverse events, serious adverse events or withdrawals did not differ significantly between the five interventions, indicating comparable safety between PEF and placebo. Larger trials over longer periods and under pharmacovigilance must be conducted to validate the safety profile of PEF in the long-term.