Eficácia e Segurança de Peficitinib (ASP015K) em Pacientes com Artrite Reumatoide com Resposta Inadequada a Metotrexate: Resultados de um Estudo Fase III Randomizado, Duplo-Cego, controlado com Placebo (RAJ4) no Japão
Takeuchi T,
Tanaka Y,
Tanaka S,
Kawakami A,
Iwasaki M,
Katayama K,
Rokuda M,
Izutsu H,
Ushijima S,
Kaneko Y,
Shiomi T,
Yamada E,
van der Heijde D
Ann Rheum Dis 2019 DOI: 10.1136/annrheumdis-2019-215164
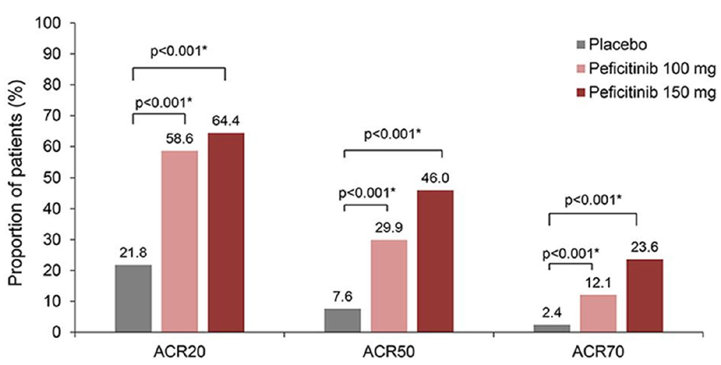
Peficitinib (PEF) 100 and 150 mg demonstrated robust clinical and structural efficacy in patients with RA who have an inadequate response to MTX. In Japan, two JAK inhibitors, TOF and BARI are currently available for RA patients with an inadequate response to conventional therapies. This randomized phase 3 study (RAJ4), assessed the efficacy and safety of two PEF doses in combination with MTX compared to PBO, in Japanese MTX-IR. Patients were randomized 1:1:1 to PBO, PEF 100 mg and 150 mg with MTX for 52 weeks. At Wk12, non-responders on PBO were switched to PEF 100 or 150 mg until the end of treatment. The remaining patients were switched to PEF at Wk28. Primary endpoints were ACR20 at Wk12/early termination (ET) and change from baseline in mTSS at Wk28/ET. Significantly more PEF (58.6%, 100 mg and 64.4%, 150 mg) treated patients compared to PBO (21.8%) achieved ACR20 responses at Wk12/ET. Mean changes from baseline at Wk28/ET occurred significantly lower in mTSS in PEF (1.62, 100 mg; 1.03, 150 mg) than PBO (3.37) recipients, along with improvements in DAS28-CRP and HAQ-DI. At Wk16-32, all ACR response rates improved after switching from PBO to PEF and were maintained through Wk52. PEF was associated with higher incidence of SIs and HZ-related diseases versus PBO.PEF demonstrated significant superiority versus PBO in reducing RA symptoms and supressing joint destruction in Japanese RA patients with inadequate response to MTX, along with an acceptable safety and tolerability profile. PEF may therefore be a valuable treatment option especially for RA patients unresponsive to conventional treatments.