A Pooled analysis of the Safety of Tofacitinib as Monotherapy or in Combination with Background Conventional Synthetic Disease-Modifying Antirheumatic Drugs in a Phase 3 Rheumatoid Arthritis Population
Kivitz AJ,
Cohen S,
Keystone E,
van Vollenhoven RF,
Haraoui B,
Kaine J,
Fan H,
Connell CA,
Bananis E,
Takiya L,
Fleischmann R
Semin Arthritis Rheum. 2018 Dec;48(3):406-415. doi: 10.1016/j.semarthrit.2018.07.006.
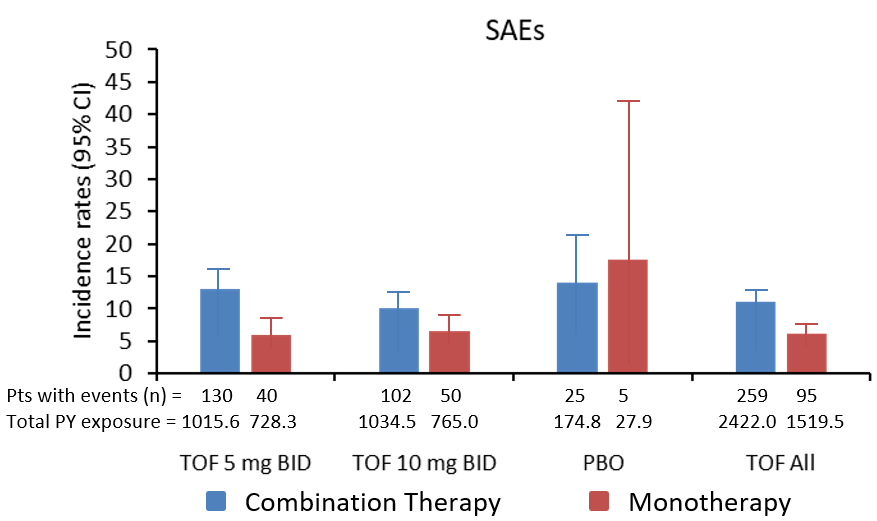
In this pooled analysis of Phase 3 Tofacitinib (TOF) trials, safety profiles were generally similar between patients receiving monotherapy and combination therapy. Although selected adverse events of interest showed lower incidence rates (IRs) for TOF monotherapy patients.TOF has been studied as monotherapy and in combination with csDMARDs. In this post-hoc analysis, data were pooled from six Phase 3 TOF studies in RA patients to further examine the safety profile when used as monotherapy or in combination with csDMARDs.Pooled data in RA patients were stratified by administration of TOF as monotherapy and (ORAL Solo and ORAL Start) or in combination with csDMARDs (ORAL Sync, ORAL Standard, ORAL Scan and ORAL Step) and by glucocorticoid use at baseline. Safety assessments included IRs for serious adverse events (SAEs), discontinuations due to AEs, serious infection events (SIEs) and herpes zoster (HZ). Baseline changes in selected laboratory parameters of interest: LDL, HDL, total cholesterol, triglycerides, haemoglobin, neutrophils, and lymphocytes were evaluated at month 3 through month 12.Safety profiles were generally similar between patients receiving monotherapy and combination therapy. Patients receiving TOF as monotherapy had numerically lower IRs for selected AEs of interest (SAEs, discontinuations due to AEs, HZ and SIEs) compared to the combination therapy, irrespective of TOF dose or glucocorticoid use. Patients receiving monotherapy had slightly numerically higher mean changes from baseline in total cholesterol, compared with its respective combination therapy. However, selected safety events of interest (HZ and SIEs) showed lower IRs (with non-overlapping 95% confidence intervals) for TOF all monotherapy, verses combination therapy. TOF monotherapy may, therefore, have fewer safety events compared with combination therapy, and have a favourable risk-benefit profile in patients with active RA who are intolerant to csDMARDs.