Efficacy and Safety of Filgotinib, a Selective Janus Kinase 1 Inhibitor, in Patients with Active Psoriatic Arthritis (EQUATOR): Results from a Randomised, Placebo-controlled, Phase 2 Trial
Mease P,
Coates LC,
Helliwell PS,
Stanislavchuk M,
Rychlewska-Hanczewska A,
Dudek A,
Abi-Saab W,
Tasset C,
Meuleners L,
Harrison P,
Besuyen R,
Van der Aa A,
Mozafarian N,
Greer JM,
Kunder R,
Van den Bosch F,
Gladman DD
Lancet. 2018 Dec 1;392(10162):2367-2377. DOI 10.1016/ S0140-6736(18)32483-8
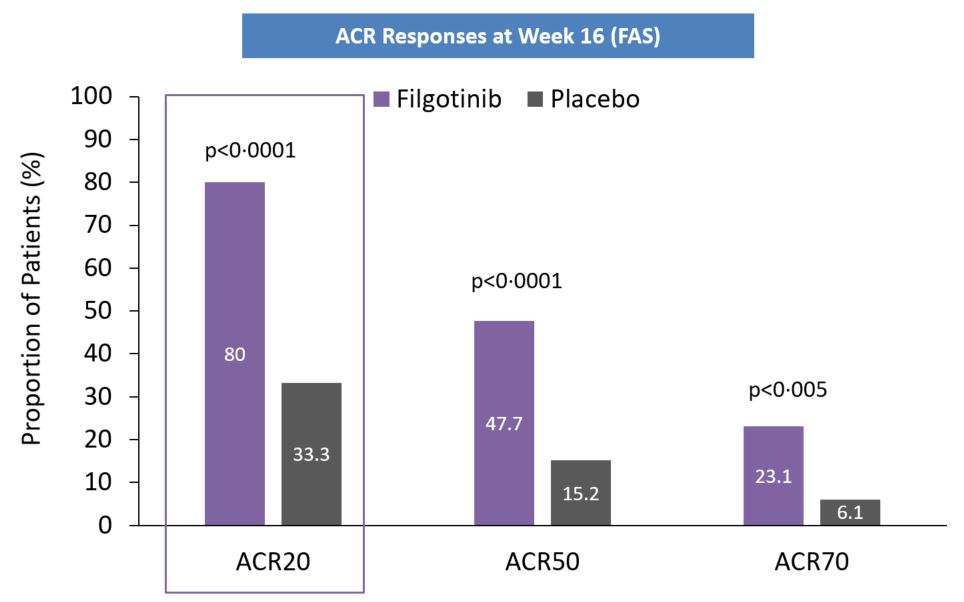
In this first clinical trial of filgotinib in patients with PsA, filgotinib was effective in treating the signs and symptoms of active PsA across various disease manifestations.The EQUATOR trial was a randomized, double-blind, placebo-controlled, Phase 2 trial, that enrolled 191 adult patients from 25 sites in seven countries. Patients with active moderate-to-severe PsA and an insufficient response or intolerance to at least one csDMARD were assigned 1:1 to receive filgotinib 200 mg or placebo orally once daily for 16 weeks. Patients continued to take csDMARDs during the study if they had received this treatment for at least 12 weeks before screening and were on a stable dose for at least 4 weeks before baseline. Following 16 weeks of treatment, a significantly greater portion of patients in the filgotinib group achieved ACR20 response, than in the placebo group. Compared with placebo, filgotinib significantly improved the signs and symptoms of peripheral arthritis, enthesitis, and psoriasis, and overall PsA disease control (according to PASDAS and fulfilment of minimal disease activity criteria). Filgotinib had a beneficial effect on patient¬ reported outcomes of physical functioning, fatigue, and pain, with significant improvements in PsA related pain intensity at week 1 and in HAQ-¬DI at week 2.Filgotinib was well tolerated after 16 weeks of treatment and associated with mostly mild or moderate adverse events. Overall, the safety profile was similar to previous reports and no new safety signals were identified. The study supports the development of filgotinib for the treatment of PsA in patients with an inadequate response to conventional synthetic DMARDs.