Tofacitinib dans la polyarthrite rhumatoïde: le manque de changement précoce dans l'activité de la maladie prédit une faible probabilité d’atteindre une activité faible de la maladie au 6ème mois
Arthritis Care Res (Hoboken) 2019 Jan;71(1):71-79. DOI: 10.1002/acr.23585
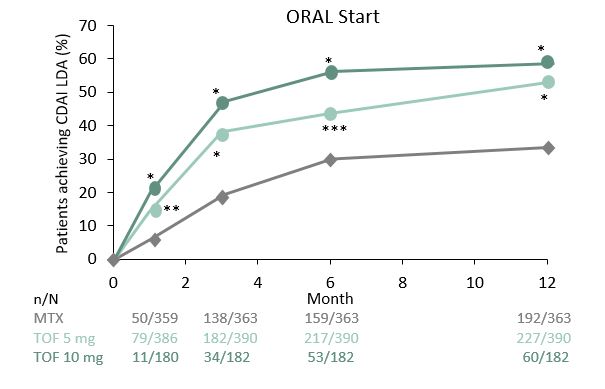
This post-hoc analysis of two, Phase 3 studies, ORAL Start and ORAL Standard shows that early treatment response can predict long-term disease activity outcomes. EULAR recommendations suggest that treat-to-target strategies require regular target assessments with treatment approaches changed if targets are not reached at 6 months. To optimize this strategy, therapy outcomes should be known, and the relationship between short and long-term outcomes defined. The current analysis focused on the disease activity outcomes in patients given tofacitinib (TOF) versus methotrexate (MTX) or adalimumab (ADA). ORAL Start¹ was a 24-month TOF study in MTX-naïve patients, and ORAL Standard² was a 12-month TOF study in MTX-IR patients. Patients achieving CDAI and DAS28-ESR low disease activity (LDA) and remission rates were recorded periodically over 1 year of each study. Probabilities for achieving LDA and remission were calculated for both studies. At 6 months, a significantly greater proportion of patients achieved CDAI LDA when given TOF versus MTX or PBO. Over 85% of patients given TOF 5 mg BID who failed to achieve improvement thresholds at 3 months, failed to achieve LDA or remission at 6 months or 1 year. Slightly lower percentage values were observed for TOF 10 mg BID not achieving LDA at 3 months and failing to achieve them at later time points. Results suggested that achievement responses at 3 months were more predictive of long-term responses compared with 1-month achievement responses. The authors found that early treatment response predicted long-term patient outcomes, with early response patterns proposed as a factor to be considered when assessing treatment continuation with TOF.1. Lee EB, et al. N Engl J Med 2014;370:2377–86 2. Van Vollenhoven RF, et al. N Engl J Med 2012;367:508–19