Effects of fostamatinib (R788), an oral spleen tyrosine kinase inhibitor, on health-related quality of life in patients with active rheumatoid arthritis: analyses of patient-reported outcomes from a randomized, double-blind, placebo-controlled trial
The Journal of Rheumatology 2013; 40(3):369-78
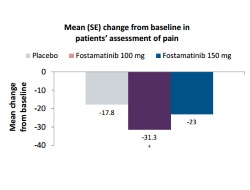
This phase 2 clinical trial assessed the influence of fostamatinib on patient reported outcomes (PROs) in 457 patients with active rheumatoid arthritis (RA) and an inadequate response to methotrexate (MTX). Patients received either placebo or fostamatinib 100 mg twice daily or 150 mg once daily (1:1:1) for 24 weeks in addition to their baseline MTX. Patients taking fostamatinib 100 mg twice daily had statistically significant improvements in health-related quality of life scores for pain, patient global assessment, physical function, fatigue and the physical component summary of Short-form 36 (SF-36) compared with placebo. For the fostamatinib 150 mg once daily group, there were greater improvements in some PROs such as physical function compared with placebo. Based on the findings of this study, additional studies are necessary to fully characterise the effect of fostamatinib on PRO in patients with RA.