An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents
Arthritis & Rheumatism 2011; 63(2):337-45
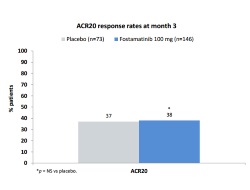
This was the first phase 2 study to be published investigating the efficacy and safety of the spleen kinase (Syk) inhibitor R788 (fostamatinib) in patients with refractory rheumatoid arthritis (RA). In this multicentre, randomised, double-blind, placebo-controlled, 3-month trial, patients with active RA on stable background treatment (excluding biologics) were randomised to receive 100 mg R788 or placebo twice daily. Differences in ACR20 responses were significant at week 6 (p=0.003), but not thereafter due to an increasing placebo response. At month 3, R788 did not result in significant improvements in ACR20 (placebo 37% vs R788 38%), ACR50 (12% vs 22%) or ACR70 responses (5% vs 9%), change in DAS28 (placebo –1.27 vs R788 –1.62) or mean change in osteitis score (+1.2 vs –0.2). However, the mean change in synovitis score was statistically greater for R788 than placebo (–0.4 vs –0.5, p=0.038). Adverse events led one patient on placebo and nine on R788 to withdraw, most commonly due to nausea and diarrhoea. There were no meaningful differences in the primary endpoint (ACR 20 response at month 3) between R788 and placebo in this group of patients in whom biologic treatments have failed. In secondary and exploratory analyses, however, differences were observed, particularly in subjects with high CRP at study initiation. The authors concluded that these findings warranted further investigation.
Slides coming soon